EC technology includes coagulation and precipitation of pollutants by the use of electricity and sacrificing electrodes for the “in situ” coagulant production. Direct current is usually applied while the use of electrodes and its characteristics (material and setup) depends on the wastewater pollution and the required effluent quality. Usually, several electrodes of the same material are used to form an electrode cell.
The EC process takes place in the EC reactor, usually consisting of a water tank and one or more electrode cells. The wastewater flows between electrodes while the direct current is applied to them. Electrodes are usually made of metal, usually iron (Fe) or aluminium (Al), to induce the electrochemical reactions needed to achieve the coagulation/flotation.
When the current is applied, metal hydroxides are produced in the reaction between either Al3+ or Fe2+ ions, created in electrolytic oxidation of Al or Fe electrodes, and OH– ions from a reduction reaction taking place on the cathode where hydrogen gas is also released according to:
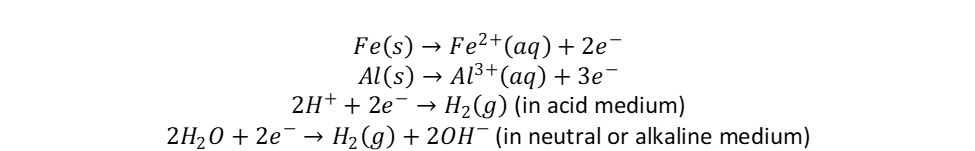
Metal hydroxides, used in the precipitation process, are produced according to the equations below:
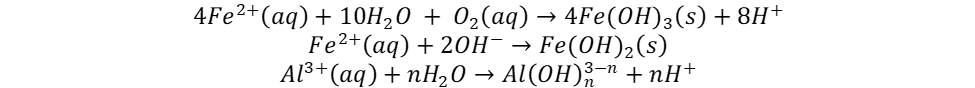
Depending on the pH of wastewater and the oxidation potential, Fe can form divalent or trivalent cations, but Al is only formed as trivalent cation. These hydroxides trap colloidal particles and create flocs which can be easily removed from water by sedimentation or flotation. The evolution of hydrogen bubbles leads to an increase in pH and it also helps flocculated particles to float out of the water.
